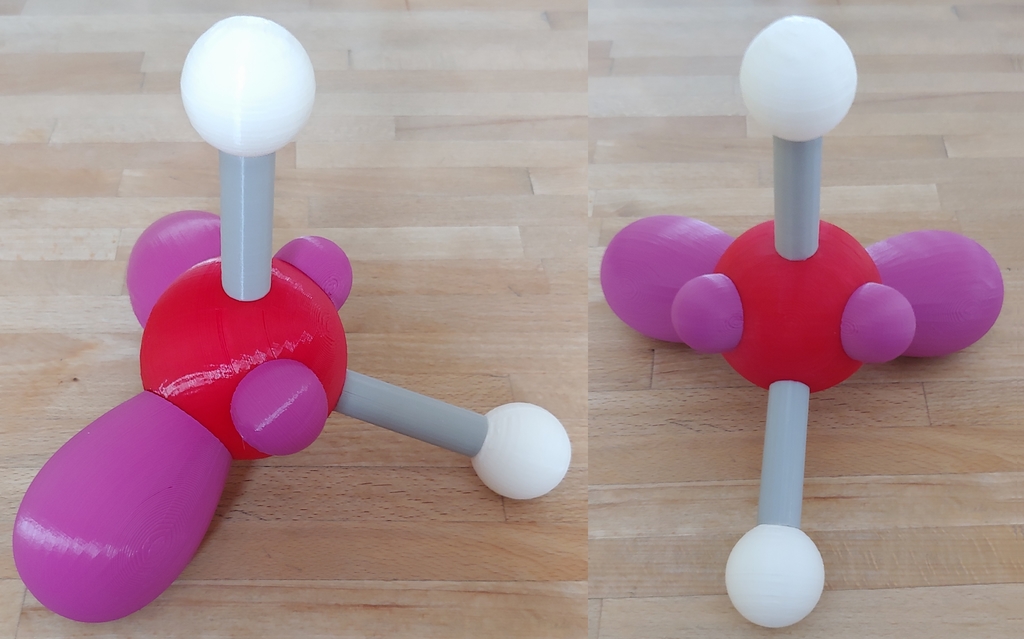
Water molecule with lone pairs of electrons
thingiverse
Following the rules of VSEPR theory, understanding why water molecules have a bent coordination geometry can be quite challenging. Typically, representations show a central atom and two ligands; lone pairs are usually left out. According to VESPR theory, it should be linear. In this model, orbitals of lone pairs are shown, demonstrating that the arrangement of four electron pairs on oxygen is almost tetrahedral. The stronger repulsive effect of lone electron pairs on bonding electron pairs causes the bond angle between these pairs to be only 104.45 degrees. This model fits a set of molecular models (methane, ammonia, and water) intended for supporting explanations of molecular structures with VESPR theory. Due to their size, they are suitable for presentations. Two different models can be built upon download: a "normal" model without lone pairs and a model with lone pairs. The bond angle and spacing are to scale, and the covalent radii of atoms have been halved for better visibility; however, the correct size ratio between oxygen and hydrogen remains intact.
With this file you will be able to print Water molecule with lone pairs of electrons with your 3D printer. Click on the button and save the file on your computer to work, edit or customize your design. You can also find more 3D designs for printers on Water molecule with lone pairs of electrons.
