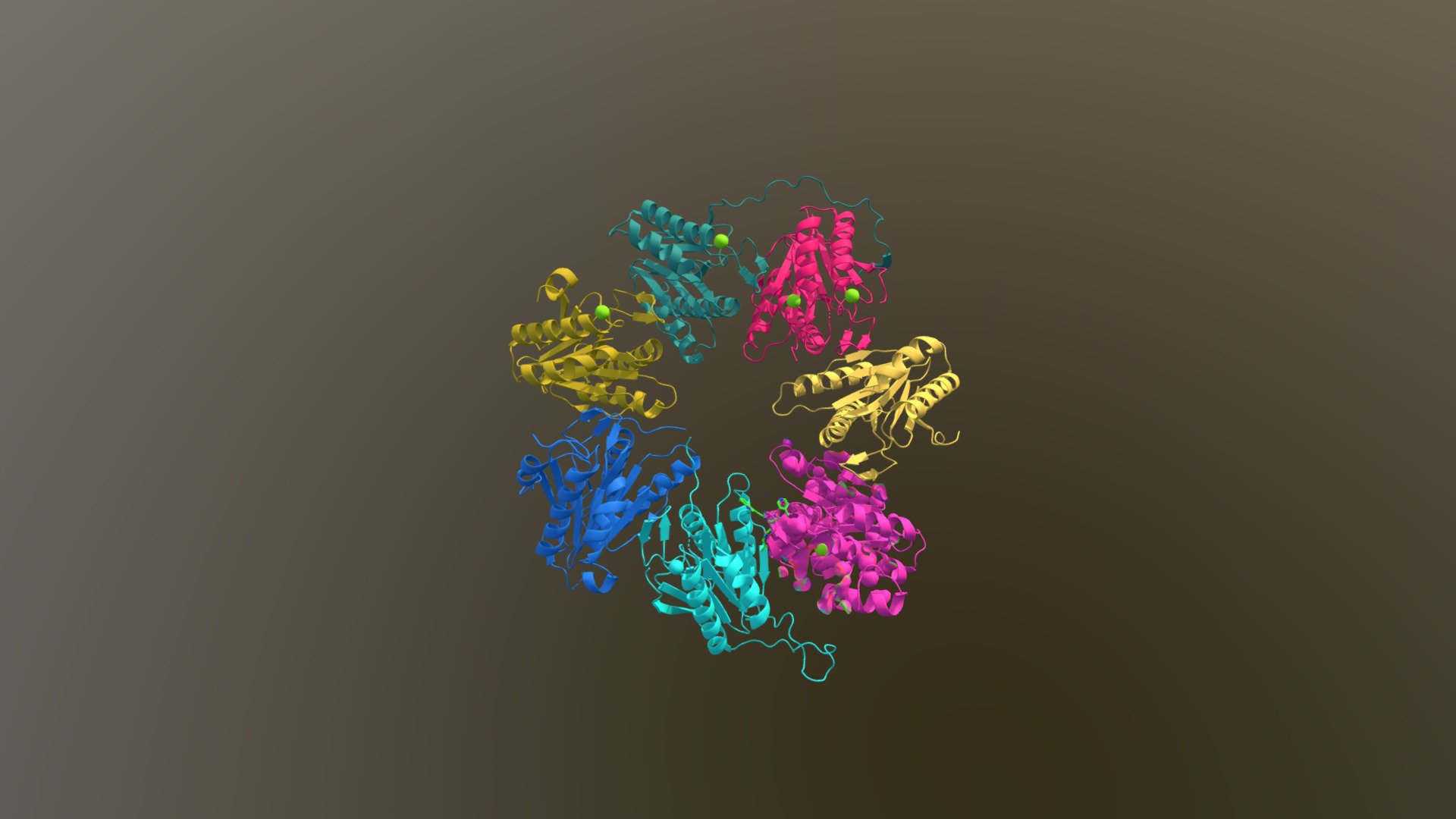
Compound 16 interaction with 20s B5 sub-unit
sketchfab
The proteasome's role in intracellular degradation spans multiple pathways, encompassing signaling, apoptosis, and inflammatory responses. A diverse array of synthetic and natural inhibitors can bind to the beta 5 sub-unit, exhibiting chymotrypsin-like mechanisms; however, their "warhead" structures often lack specificity, are highly reactive, and exhibit instability. Compound 16, demonstrated here in non-covalent interaction with the B5 subunit of a beta ring, is a novel compound engineered through sequential optimization of S-homo-phenylalanine to target chymotrypsin-like mechanisms. Compound 16 forms an optimal interaction at the S3 binding pocket accompanied by weaker S1 binding pocket and sub-pocket interactions. This compound displays higher affinity for B5 (Ki=1.2nM) compared to the covalent inhibitor Bortezomib (Ki=0.56nM). By exclusively inhibiting the B5 subunit, it is capable of halting cancer cell proliferation.
With this file you will be able to print Compound 16 interaction with 20s B5 sub-unit with your 3D printer. Click on the button and save the file on your computer to work, edit or customize your design. You can also find more 3D designs for printers on Compound 16 interaction with 20s B5 sub-unit.
