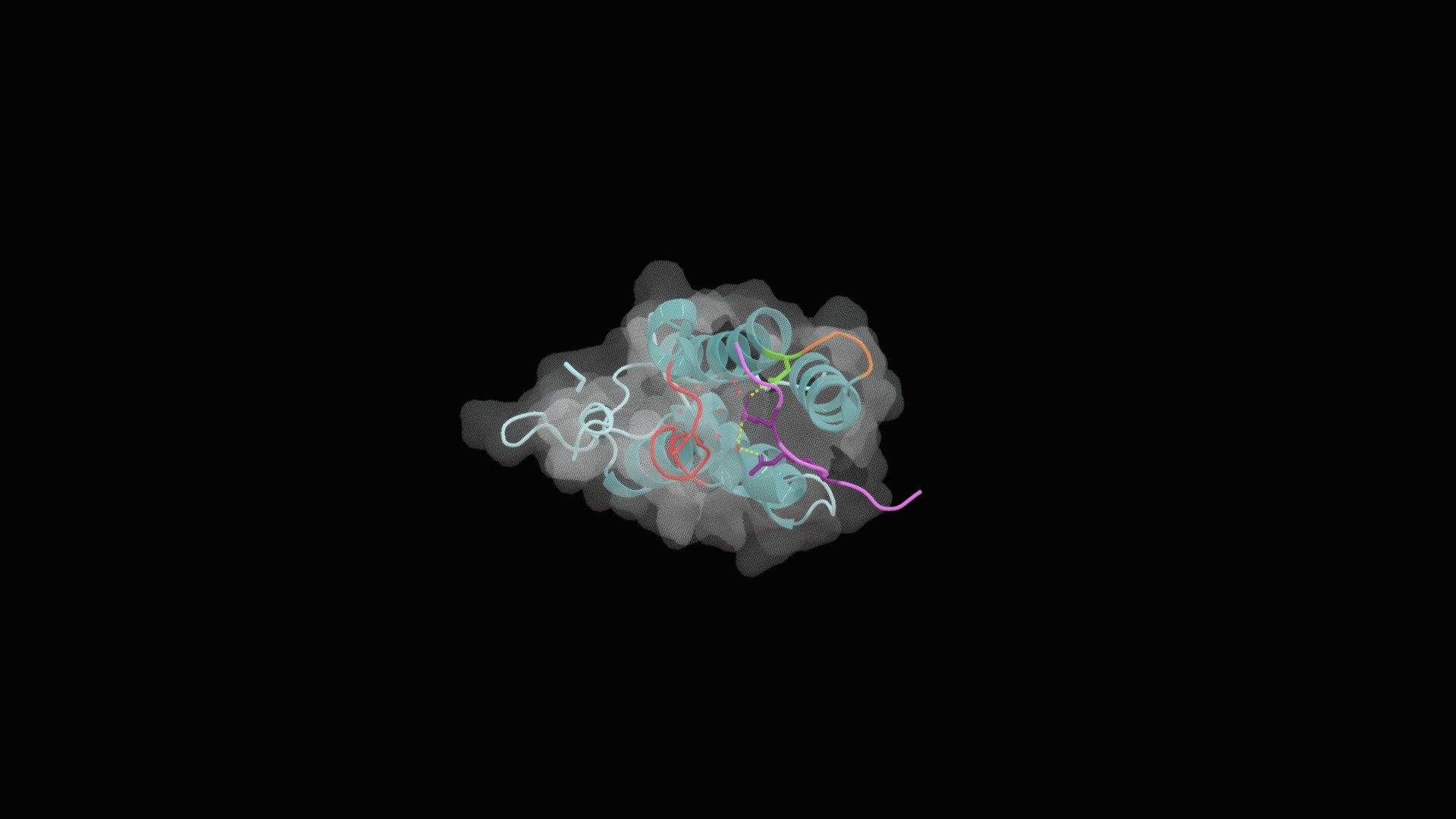
Bromodomain of BRD4 with a diacetylated peptide
sketchfab
Bromodomain protein 4 (BRD4) plays a key role in the bromodomain and extra-terminal domain (BET) family, which is characterized by its ability to associate with acetylated lysines on histone tails (1). By recognizing these epigenetic modifications, BRD4 recruits proteins that are essential for chromatin remodeling and activating transcription sites (2). In this context, the first bromodomain (BD1) of BRD4 forms a strong association with TOP2A, which is mediated by a Kac-XX-motif that resembles histone H4. The deep hydrophobic binding pocket in BD1 is highly conserved and plays a crucial role in recognizing and binding acetylated peptides (3). This interaction triggers the other domains of BRD4 to perform its reader function, allowing it to accurately recognize epigenetic modifications and facilitate chromatin remodeling (1).
With this file you will be able to print Bromodomain of BRD4 with a diacetylated peptide with your 3D printer. Click on the button and save the file on your computer to work, edit or customize your design. You can also find more 3D designs for printers on Bromodomain of BRD4 with a diacetylated peptide.
